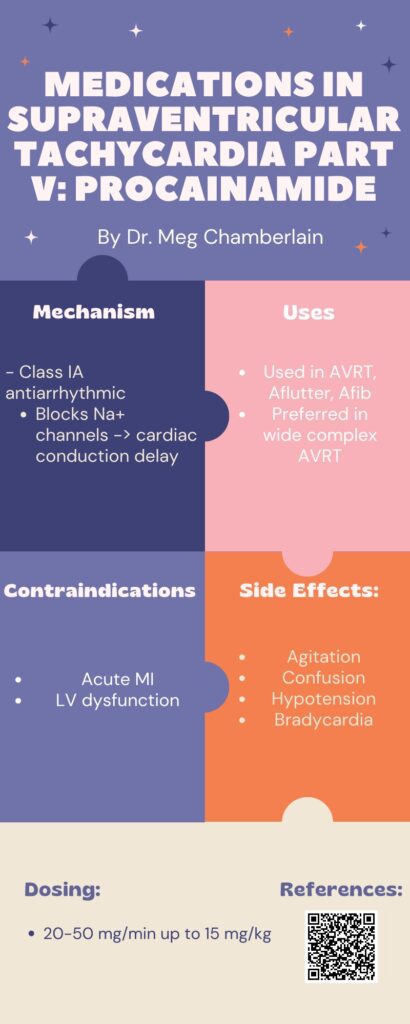
Medications in Supraventricular Tachycardia Part 5: Procainamide by Meg Chamberlain


30 year old female is brought by EMS from an outpatient surgery center for evaluation of persistent hypotension and vaginal bleeding after an elective abortion and D&E at approximately 20 weeks gestation. Initial vitals on arrival were T 98.6 F, HR 99, BP 62/palp, RR 21, O2 100%. On exam, patient was pale and lethargic but mentation intact. There is scant vaginal bleeding on pelvic exam. A bedside FAST is performed and shown below. What is the interpretation of the FAST, and which views demonstrate free fluid if present?
Answer: Free Fluid in RUQ, LUQ, and Pelvis
The patient received 2 units of uncrossed pRBCs in addition to 1g TXA IV and was taken emergently to the OR with OBGYN for exploratory laparotomy. She was found to have 1500 ccs of hemoperitoneum from an actively bleeding R uterine artery laceration. She did well post-op and was discharged a few days later!
Focused Assessment with Sonography for Trauma
Key learning point for this case: clotted blood is more hyperechoic and can start to resemble tissue or other structures. Easy to miss if not looking closely.
References:
By Dr. Jacob Martin, MD
HPI:
PE:
Vitals: BP 182/78 | Pulse 61 | Temp 97.7 °F (Oral) | Resp 22 | SpO2 100%
Ddx for Generalized Weakness:
Initial Diagnostics:

Management:
Case Progression:
Thyrotoxic Periodic Paralysis (TPP)
References:
Bilha S, Mitu O, Teodoriu L, Haba C, Preda C. Thyrotoxic Periodic Paralysis-A Misleading Challenge in the Emergency Department. Diagnostics (Basel). 2020;10(5):316. Published 2020 May 18. doi:10.3390/diagnostics10050316
Lin SH, Huang CL. Mechanism of thyrotoxic periodic paralysis. J Am Soc Nephrol. 2012;23(6):985-988. doi:10.1681/ASN.2012010046
What is cardiac tamponade?
-Cardiac tamponade is a medical or traumatic emergency that occurs when enough fluid accumulates in the pericardial sac to cause compression of the heart, leading to a decrease in cardiac output and obstructive shock.
Risk factors for tamponade:
-Besides hemorrhage (from something such as a stab wound or a left ventricular wall rupture s/p MI), other risk factors include infection (i.e., TB, myocarditis), autoimmune diseases, neoplasms, uremia, inflammatory disorder such as pericarditis.
True or false: the size of the pericardial effusion directly correlates with the risk of developing tamponade.
-FALSE! The rate at which fluid accumulates in the pericardial sac correlates with the risk of developing tamponade. The classic example is a traumatic cardiac injury which leads to hemopericardium. The rapid build-up of blood in the sac quickly leads to the inability of the chambers relax, which leads to decreased venous return, decreased diastolic filling, and decreased cardiac output.
-In situations such as neoplasms where the effusions grow at a much slower rate, there is time for the pericardial sac to stretch; these volumes can be substantially higher without causing tamponade physiology to develop.
What is one of the first compensatory vital signs seen in tamponade physiology, and also the most common EKG finding?
-Sinus tachycardia. The classic finding of electrical alternans is only present to 5-10% of cases of tamponade.

How does the patient present? What are their physical exam findings?
-Patients present with symptoms consistent with obstructive shock – lethargy, tachypnea, chest pain, palpitations. In severe cases, patients can experience dizziness, syncope, and/or altered mental status.
-Beck’s Triad: hypotension, jugular venous distention, muffled heart sounds.
-Pulsus paradoxus is defined as a decrease in systolic blood pressure of >10mmHg with inspiration. It is an important finding suggesting tamponade but may be absent in people with an elevated diastolic blood pressure, ASD, pulmonary hypertension, or aortic regurgitation.
What are ultrasound findings suggestive of cardiac tamponade?
-A plethoric IVC is the most sensitive finding of tamponade. IVC plethora is defined by a diameter equal or greater to 2 cm with less than 50% collapsibility during inspiration.
-Right ventricular free wall collapse during diastole is considered to be the most specific sonographic finding of tamponade. RV free wall collapse can also be used as a measurement of severity. Initially, collapse of the RV free wall will only be present during expiration, but as the pressure increases, detection is possible throughout the respiratory cycle.
-Right atrial collapse (most often during systole, when the intra-atrial pressure is low) is often observed before right ventricular collapse. RA collapse longer than 1/3 of the total cardiac cycle has been described as an 100% sensitive and specific finding of tamponade.
Sources
Stashko E, Meer JM. Cardiac Tamponade. [Updated 2021 Aug 11]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK431090/
Kalter HH, Schwartz ML. Electrical alternans. NY State J Med. 1948;1:1164-66.
Pérez-Casares, Alejandro et al. “Echocardiographic Evaluation of Pericardial Effusion and Cardiac Tamponade.” Frontiers in pediatrics vol. 5 79. 24 Apr. 2017, doi:10.3389/fped.2017.00079
Mugmon, Marc. “Electrical alternans vs. pseudoelectrical alternans.” Journal of community hospital internal medicine perspectives vol. 2,1 10.3402/jchimp.v2i1.17610. 30 Apr. 2012, doi:10.3402/jchimp.v2i1.17610
A 4-month old female born at full term otherwise healthy presents to the ED after parents observed her “turning blue” and “breathing funny” for less than a minute that spontaneously self-resolved. Parents report no recent fever or illness and say this has never happened before. Upon arrival to the ED patient appears to be well appearing and in no acute distress, afebrile and with reassuring vital signs and physical exam. Parents ask if they can take her home. What do you do?
A. Tell the parents the baby needs to be admitted to the pediatric floor
B. Monitor the baby on pulse oximetry for another 2 hours and then discuss possible discharge with the parents
C. Tell the parents the baby is fine and discharge to home
D. Tell the parents the baby needs to be admitted to the PICU
Answer: Monitor the baby on pulse oximetry for another 2 hours and then discuss possible discharge with the parents
This baby presents with a BRUE, a Brief Resolved Unexplained Event (formerly known as ALTE, Apparent Life-Threatening Event) as defined by:
And according to the most recent American Academy of Pediatrics guidelines, this patient is considered low risk according to the following criteria:
Therefore, this low risk patient may be safely discharged home with close pediatrician follow up after a period of observation and education provided to the parents about BRUEs. This is different than past practice where nearly all patients with BRUEs (then called ALTEs) were hospitalized. It should be noted that BRUEs can be related to a range of conditions both benign and more concerning. Possible etiologies include GERD, breath-holding spells, non-accidental trauma, and serious bacterial infection. The risk of a serious disorder presenting as a BRUE is unknown, therefore a thorough history and physical exam is essential.
References:
Joel S. Tieder, Joshua L. Bonkowsky, Ruth A. Etzel, Wayne H. Franklin, David A. Gremse, Bruce Herman, Eliot S. Katz, Leonard R. Krilov, J. Lawrence Merritt, Chuck Norlin, Jack Percelay, Robert E. Sapién, Richard N. Shiffman, Michael B.H. Smith, for the SUBCOMMITTEE ON APPARENT LIFE THREATENING EVENTS, Pediatrics May 2016, 137 (5) e20160590; DOI: 10.1542/peds.2016-0590

A 31 yo male presents with left thumb pain after a dirt bike crash. Patient is unable to move his left thumb and has tenderness at the base. An x-ray is shown below. What’s the diagnosis?

Answer: Type I first metacarpal fracture (Bennett fracture)
Classification of first metacarpal base fractures
References:
Stapczynski, J. Stephan,, and Judith E. Tintinalli. Tintinalli’s Emergency Medicine: A Comprehensive Study Guide. 7th ed. New York, N.Y.: McGraw-Hill Education LLC., 2011.
by Edward Guo M.D.
HPI
A 70 year old male with a past medical history of hypertension, type 2 diabetes, and atrial fibrillation on warfarin presents for visual changes. He is accompanied by his daughter who states that about one hour ago, his vision on the right side became blurry. There is associated right facial numbness and headache. His daughter believes that he has become more confused over this time period. Fingerstick glucose is 220. An EKG is obtained which shows atrial fibrillation at a rate of 92.
Exam
BP 151/75, HR 92, T 97.8F, RR 18, SpO2 98%
Comfortable appearing in no acute distress. GCS E4 V4 M6. No facial droop. Decreased sensation to right side of face. 5/5 strength and sensation in all extremities. No difficulty with rapid alternating movements. Extraocular motion intact. Left gaze preference with right sided homonymous hemianopia.
Differential diagnosis: acute ischemic stroke, spontaneous intracranial hemorrhage, complex migraine, toxic-metabolic encephalopathy
Case continued: Neurology is emergently consulted and a stroke alert is activated. CT/CTA of the head and neck shows no acute intracranial hemorrhage and no large vessel occlusion. Labs are notable for an INR of 1.6. The decision is made in conjunction with neurology to administer thrombolytics, and the patient is admitted to neurology critical care. Repeat head CT 24 hours later demonstrates a left parieto-occiptal infarct.
Pearls:
– This patient’s neurologic deficits including right sided facial numbness, right homonymous hemianopsia, left sided gaze preference, and aphasia localize to a cortical distribution as noted above.
– Warfarin use alone is not a contraindication to thrombolytics for acute ischemic stroke. The INR must be > 1.7 in addition to be an exclusion criterion.
– This patient had multiple previous subtherapeutic outpatient INR levels which likely precipitated an embolic stroke.
– In patients without contraindications, the decision to administer thrombolytics for acute ischemic stroke should be clinical without waiting for results of laboratory testing with the exception of a point of care glucose and patients with suspected coagulopathy.
– Other common exclusion criteria to use of thrombolytics in acute ischemic stroke include previous head trauma or stroke within 3 months, any previous intracranial hemorrhage, SBP > 185 or DBP > 110, or known intracranial mass such as neoplasm or aneurysm.
References:
Go S, Kornegay J. Stroke Syndromes. In: Tintinalli JE, Ma O, Yealy DM, Meckler GD, Stapczynski J, Cline DM, Thomas SH. eds. Tintinalli’s Emergency Medicine: A Comprehensive Study Guide, 9e.
Powers WJ, Rabinstein AA, Ackerson T, et al. 2018 Guidelines for the Early Management of Patients With Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association [published correction appears in Stroke. 2018 Mar;49(3):e138] [published correction appears in Stroke. 2018 Apr 18;:]. Stroke.
| Differential Diagnosis | Clinical Findings | Management |
| Balanoposthitis (cellulitis of glans or foreskin) | Glans, foreskin, or both are erythematous, tender, or edematous | Warm soaks +/- oral antibiotic or antifungal cream depending on etiology |
| Phimosis | Stenosis of distal foreskin preventing retraction of foreskin over the glans | Most uncircumcised infants have normal, physiologic phimosis that will spontaneously resolve by 5 years of age. Rarely requires treatment other than daily hygiene. Monitor for if foreskin completely seals off causing acute urinary retention – true emergency. |
| Paraphimosis | Entrapped ring of foreskin retracted proximal to glans of penis causing pain, erythema, and swelling | Consult pediatric urology emergently! In cases when urology is not immediately available or necrosis of penis is imminent, ED physician may attempt reduction. |
References:
– Liu DR. Pediatric Urologic and Gynecologic Disorders. In: Tintinalli JE, Ma O, Yealy DM, Meckler GD, Stapczynski J, Cline DM, Thomas SH. eds. Tintinalli’s Emergency Medicine: A Comprehensive Study Guide, 9e. McGraw Hill; 2020
– https://www.uptodate.com/contents/balanitis-and-balanoposthitis-in-children-and-adolescents-management
A 40 year old female with a history of hyperlipidemia presents for abdominal pain. She has been having intermittent pain in her right upper quadrant after meals without vomiting or change in her bowel habits. Vital signs are within normal limits. She has mild tenderness to palpation to the right upper quadrant on exam with a negative Murphy’s sign. Point of care pregnancy test is negative. Her workup including CBC, BMP, LFTs, and lipase are unremarkable. A right upper quadrant ultrasound demonstrates numerous gallstones without evidence of cholecystitis. Which of the following is recommended for first line treatment of this patient’s suspected condition?
A: Acetaminophen
B: Gabapentin
C: Ketorolac
D: Morphine
Answer: Ketorolac
This patient is presenting with biliary colic which occurs by a gallstone causing periodic obstruction of the cystic duct. Management includes symptom control and outpatient surgical referral for cholecystectomy. NSAIDs are first line therapy. When administered parenterally, NSAIDs have similar analgesic effect compared to opioids for biliary colic. In addition, NSAIDs reduce the rate of short term complications such as acute cholecystitis.
Acetaminophen is an antipyretic that has analgesic properties but is not first line for biliary colic. Gabepentin is typically used for neuropathic pain such as diabetic neuropathy or shingles. Opioids such as morphine are reserved for when NSAIDs are not effective in reducing pain but are not first line due to safety and side effects such as hypoventilation. It is known that opioids cause sphincter of Oddi spasm, but the clinical significance of this is unclear.
References:
Besinger B, Stehman CR. Pancreatitis and Cholecystitis. In: Tintinalli JE, Ma O, Yealy DM, Meckler GD, Stapczynski J, Cline DM, Thomas SH. eds. Tintinalli’s Emergency Medicine: A Comprehensive Study Guide, 9e. McGraw-Hill Education; 2020.
Colli A, Conte D, Valle SD, Sciola V, Fraquelli M: Meta-analysis: nonsteroidal anti-inflammatory drugs in biliary colic. Aliment Pharmacol Ther 35: 1370, 2012. [PubMed: 22540869]